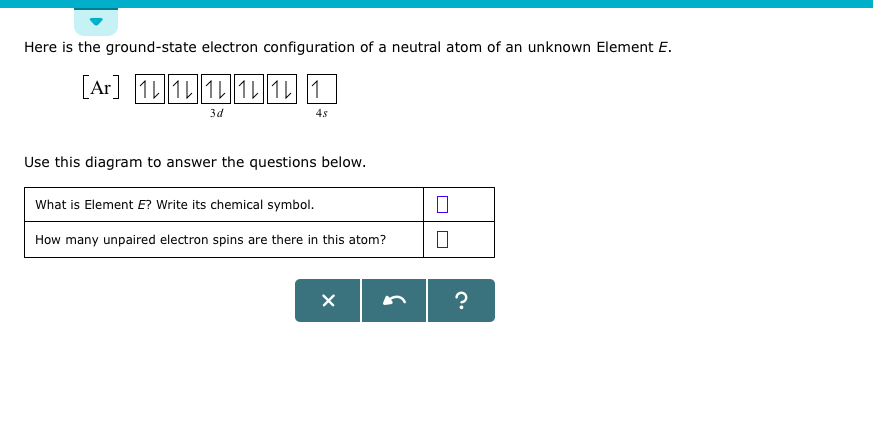
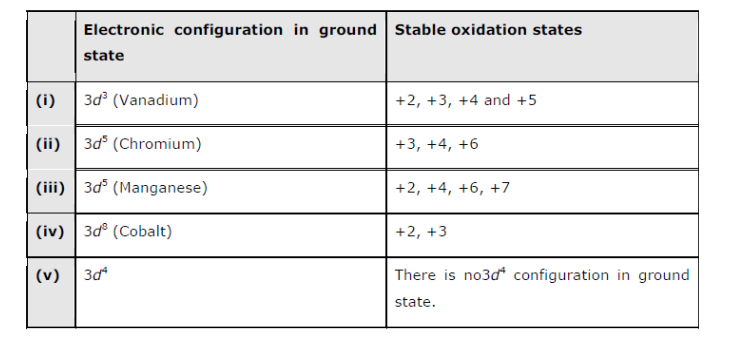
Electronic Configuration Of 3d Series Elements
These details will help you to understand the transition metals in a better manner and further enable you to delve deeper into the period table.
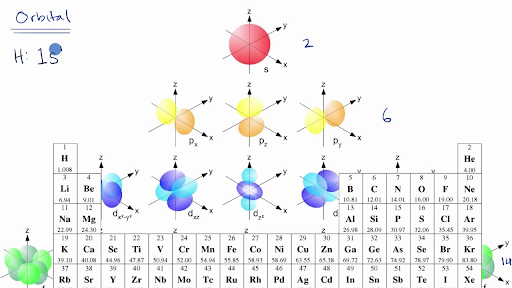
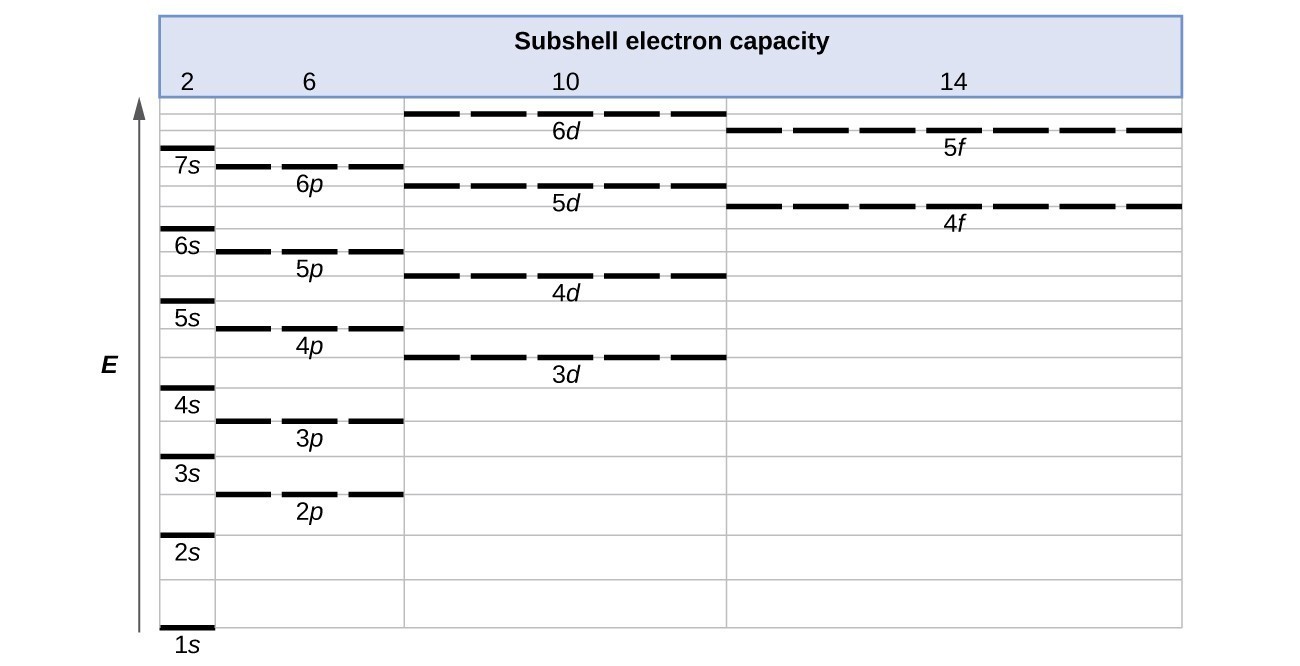
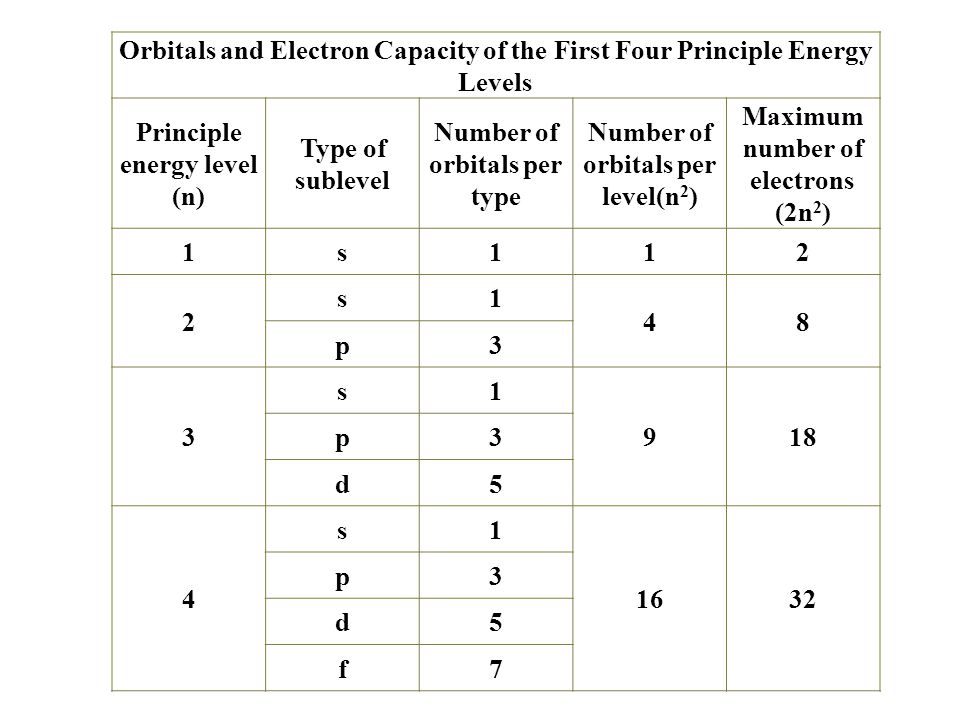
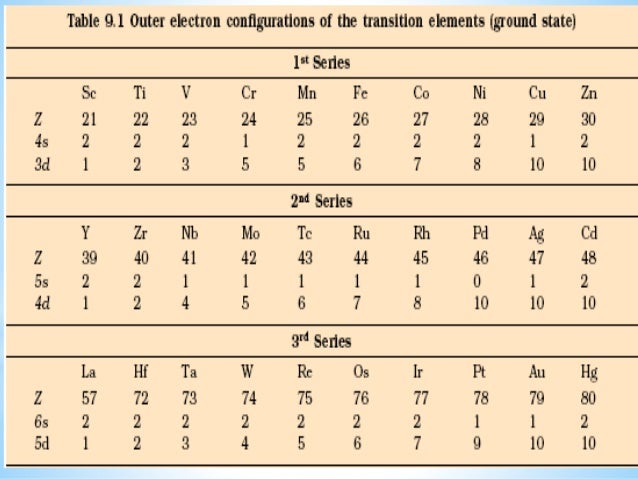
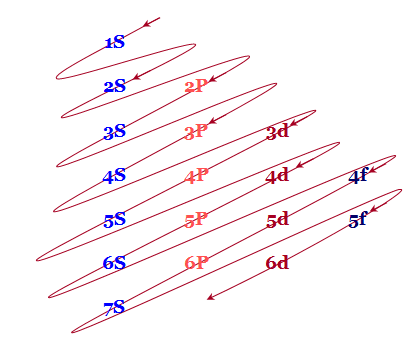
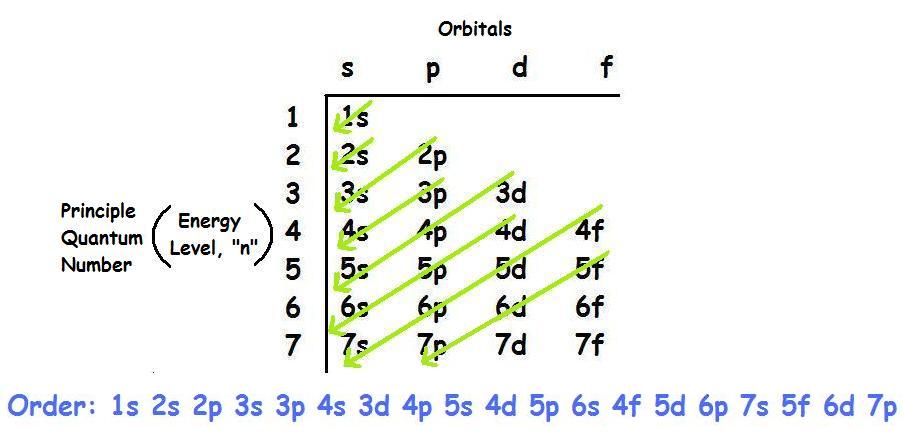
Electronic configuration of 3d series elements. The s orbital can get two electrons while p d and f orbitals can hold 6 10 and 14 electrons separately. The m shell contains 3s 3p and 3d and can carry 18 electrons. Every element has a definite number of protons which its atomic number. The n1 remains for the inward d orbitals which may have one to ten electrons and the peripheral ns orbital may have one or two electrons.
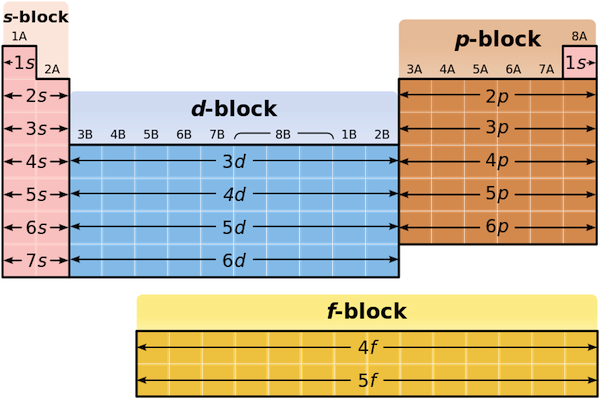
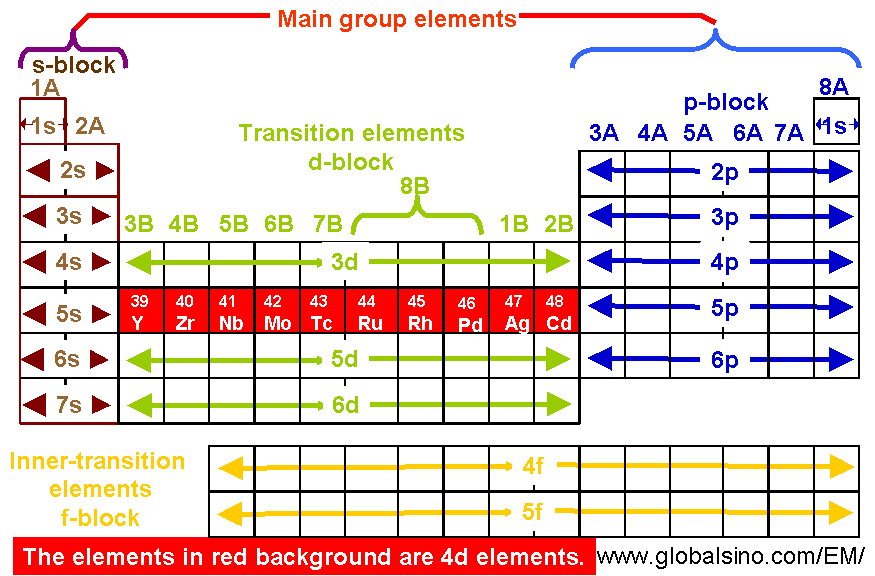
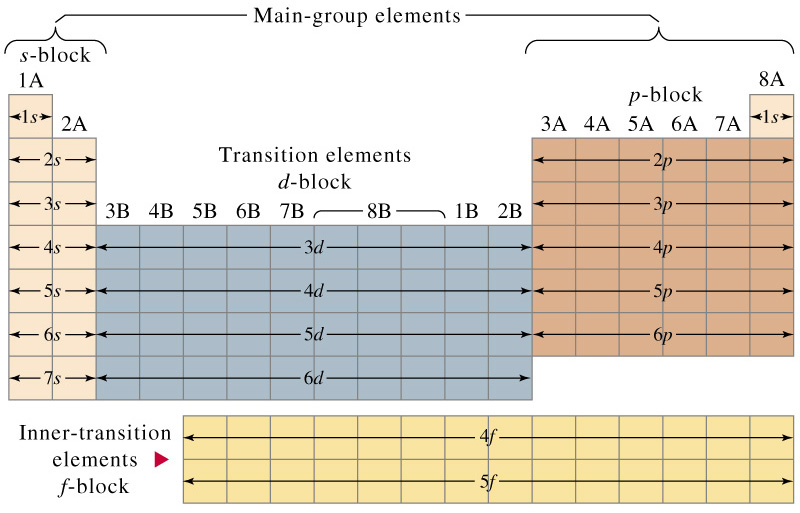
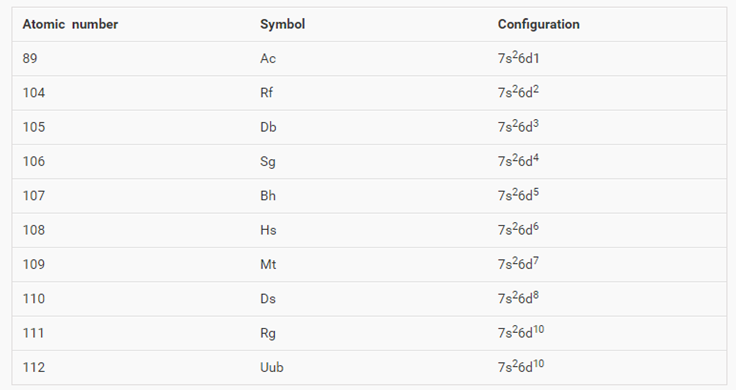
You should also go through the electronic configuration of second series third series and fourth series d block elements as it will help you to learn about a large set of elements in the d block. The dblock involves the middle area flanked by s and p blocks in the periodic table. The n shell containing 4s 4d 4p and 4f can carry 32 electrons. The n1 remains for the inward d orbitals which may have one to ten electrons and the peripheral ns orbital may have one or two electrons.
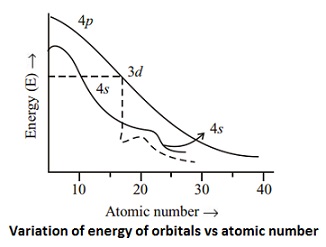
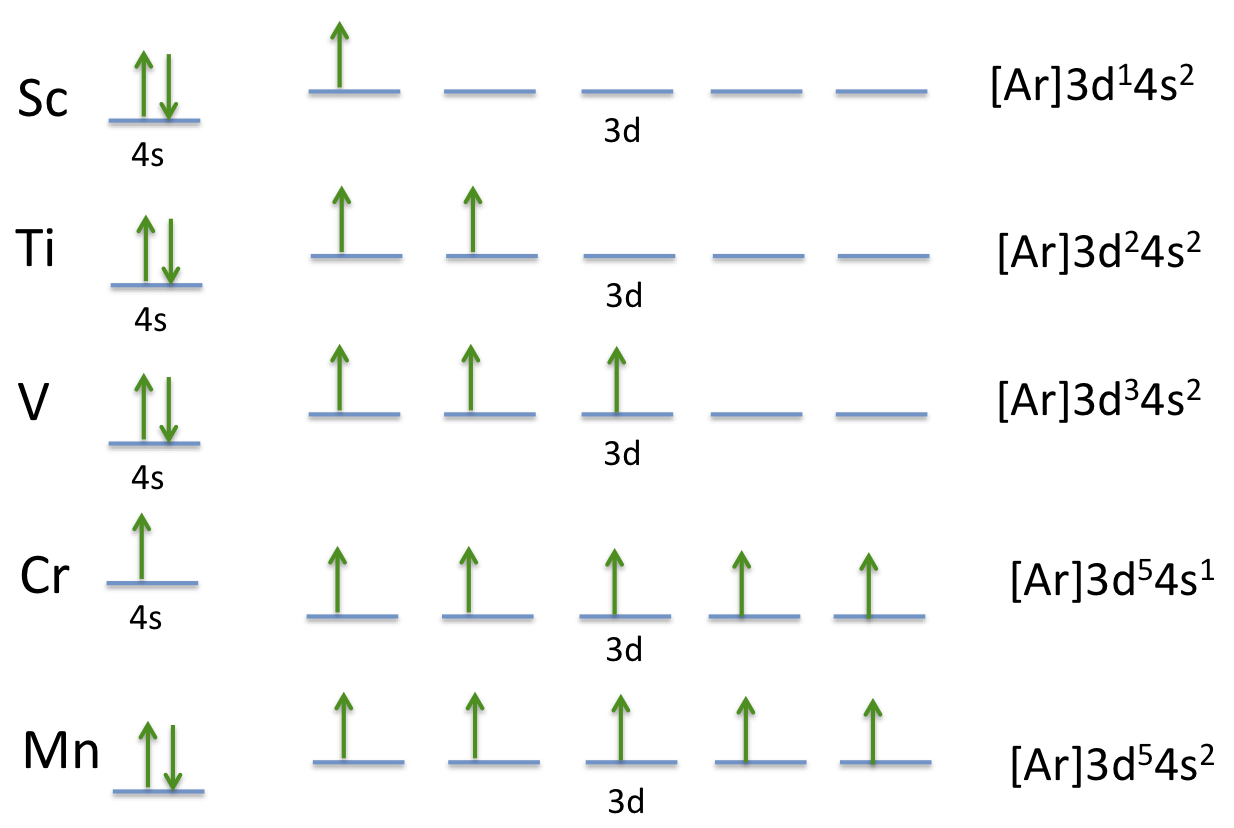
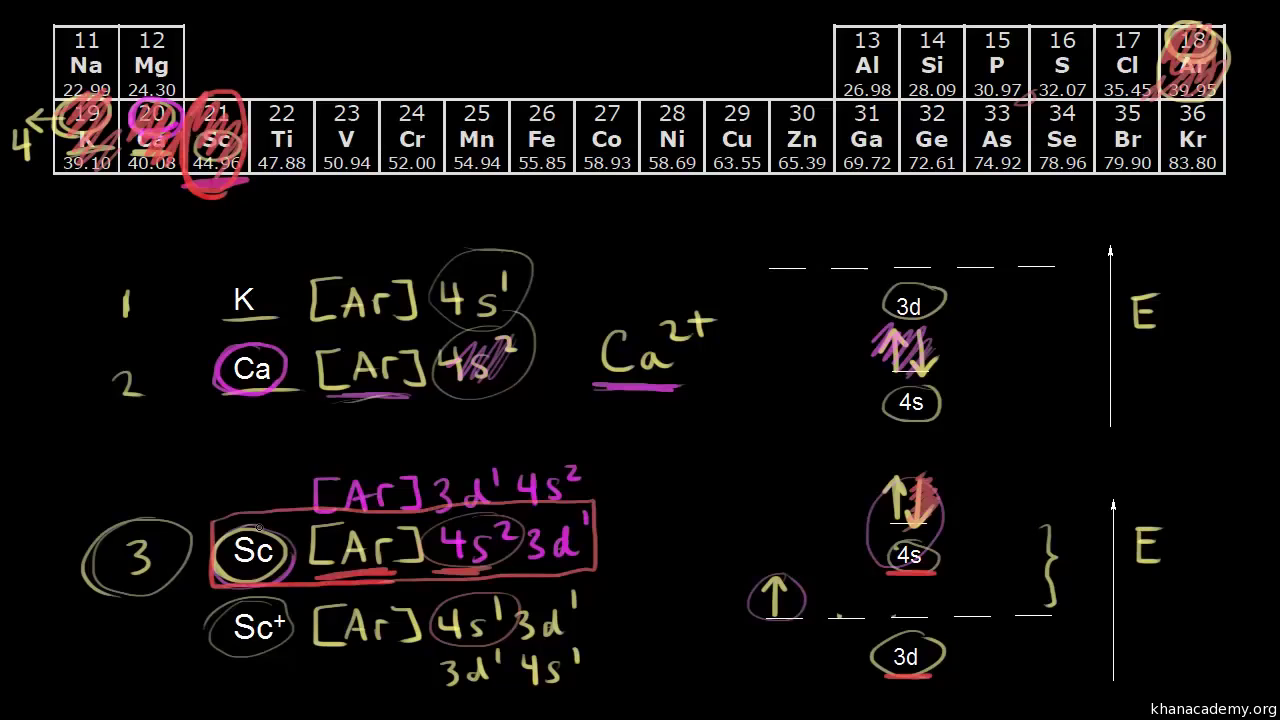
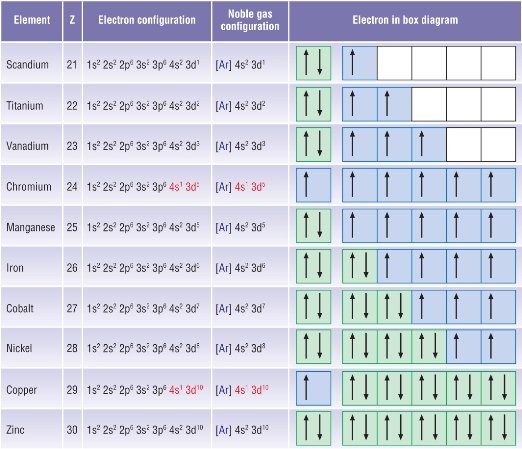
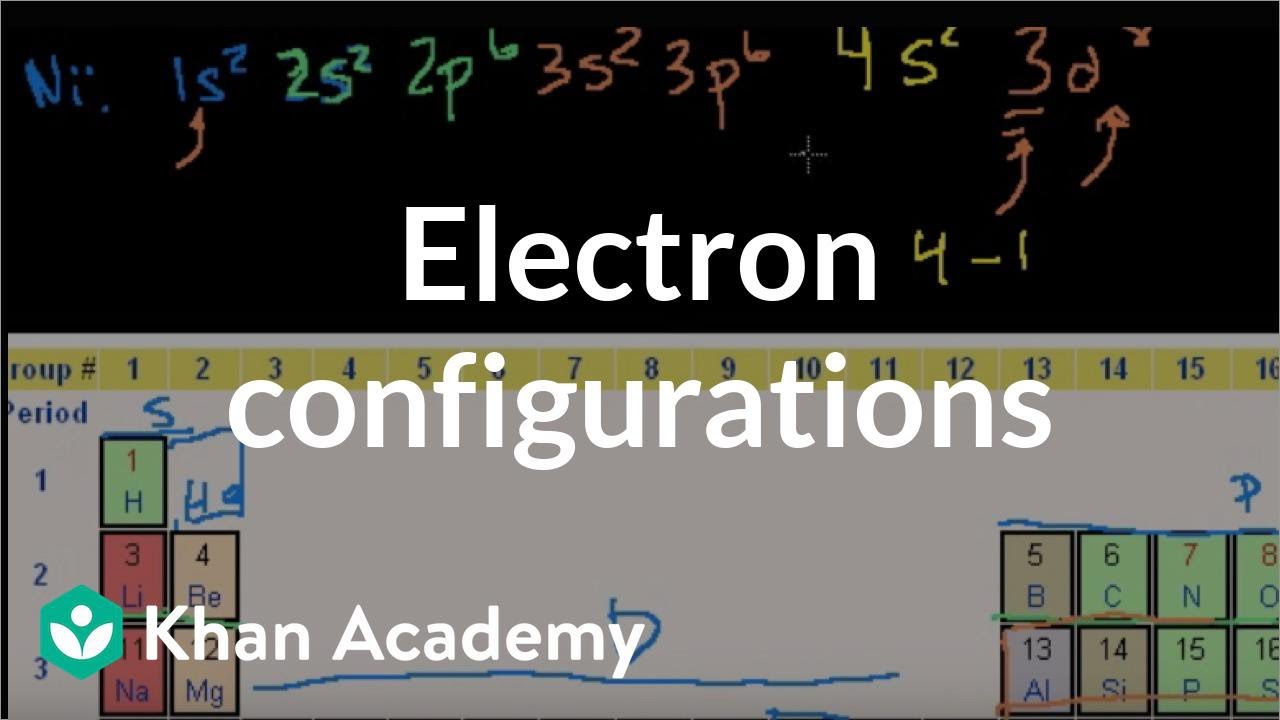
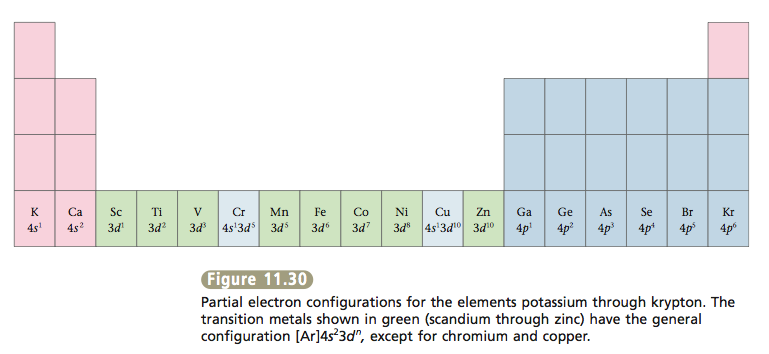
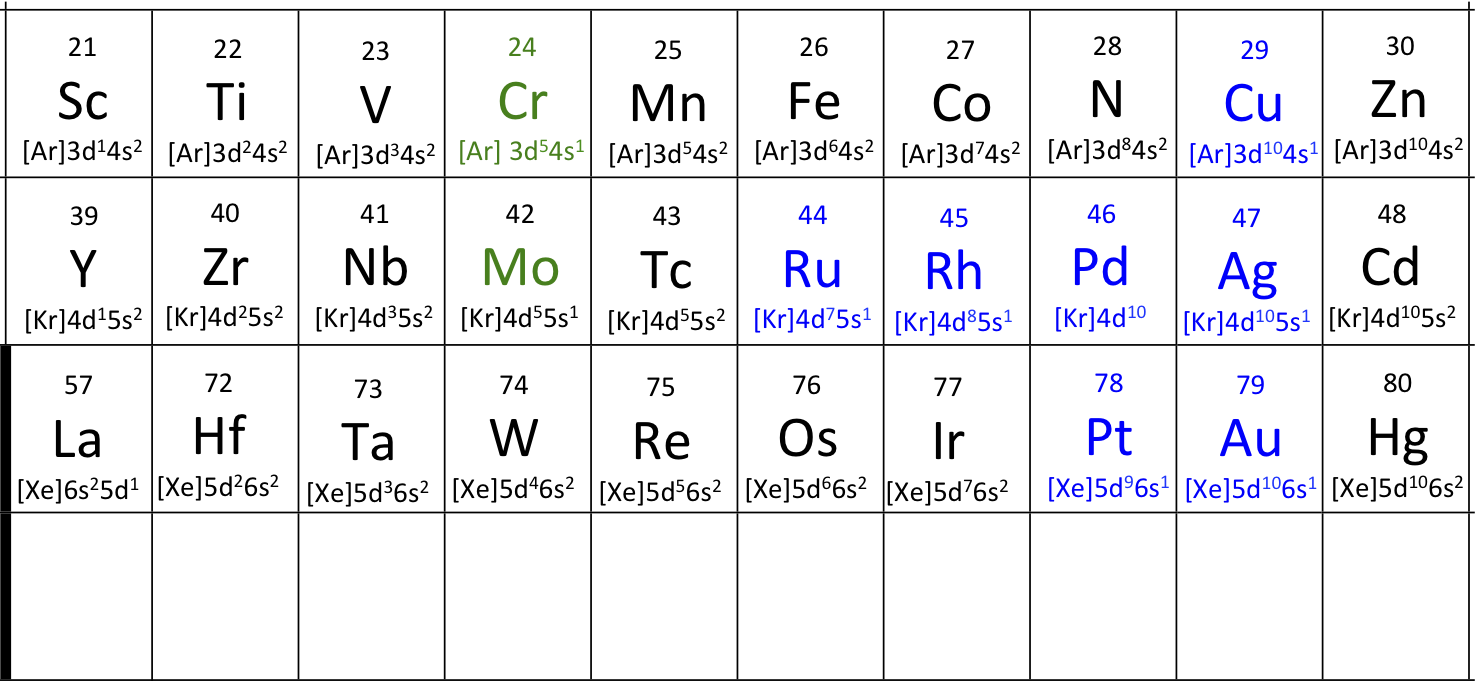
Actually two of these electrons actually move up to the higher energy orbital so two of those electrons move up to the 4s orbital here like that. This distribution is fixed for every element. Generally the electronic configuration of these elements is n 1 d 110ns 12. The number of electrons is same as the number of protons in an atom.
You might think it would be argon 3d 3 but thats not what we observed for the electron configuration for scandium. Generally the electronic configuration of these elements is n 1 d 110 ns 12.




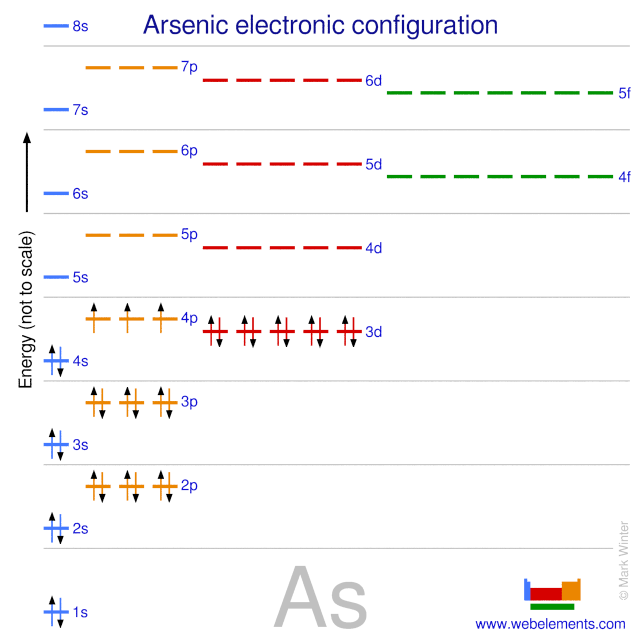











.PNG)
















.jpg?revision=1&size=bestfit&width=656&height=425)