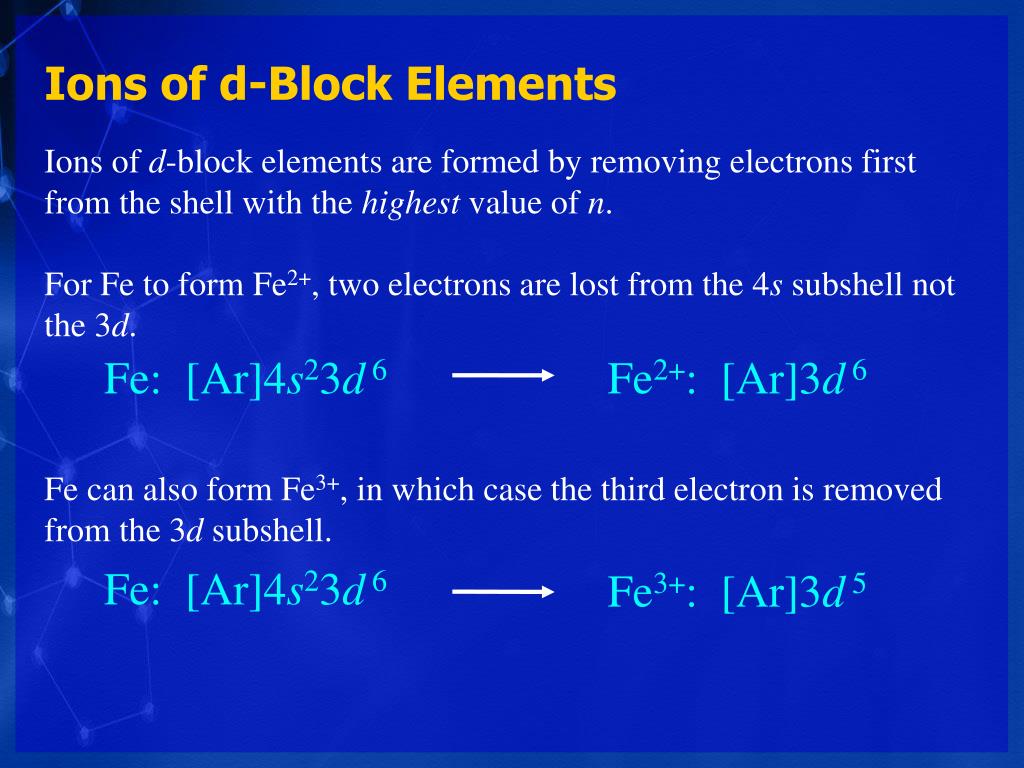
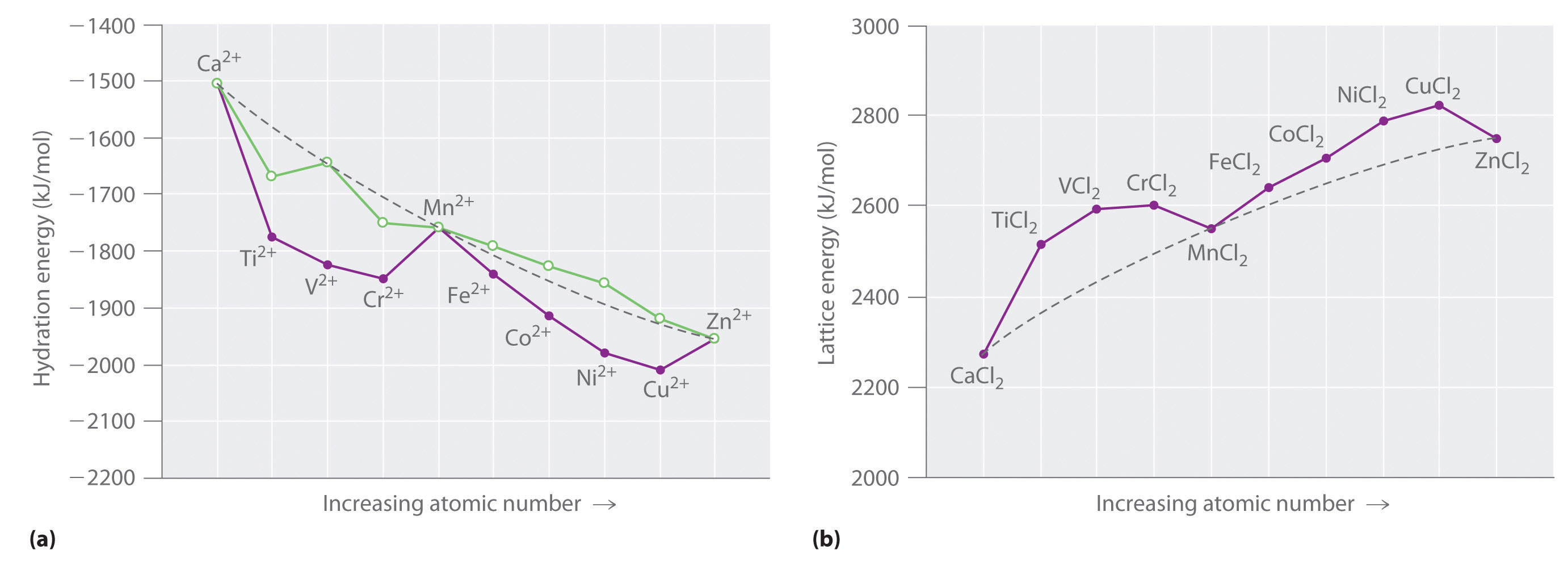
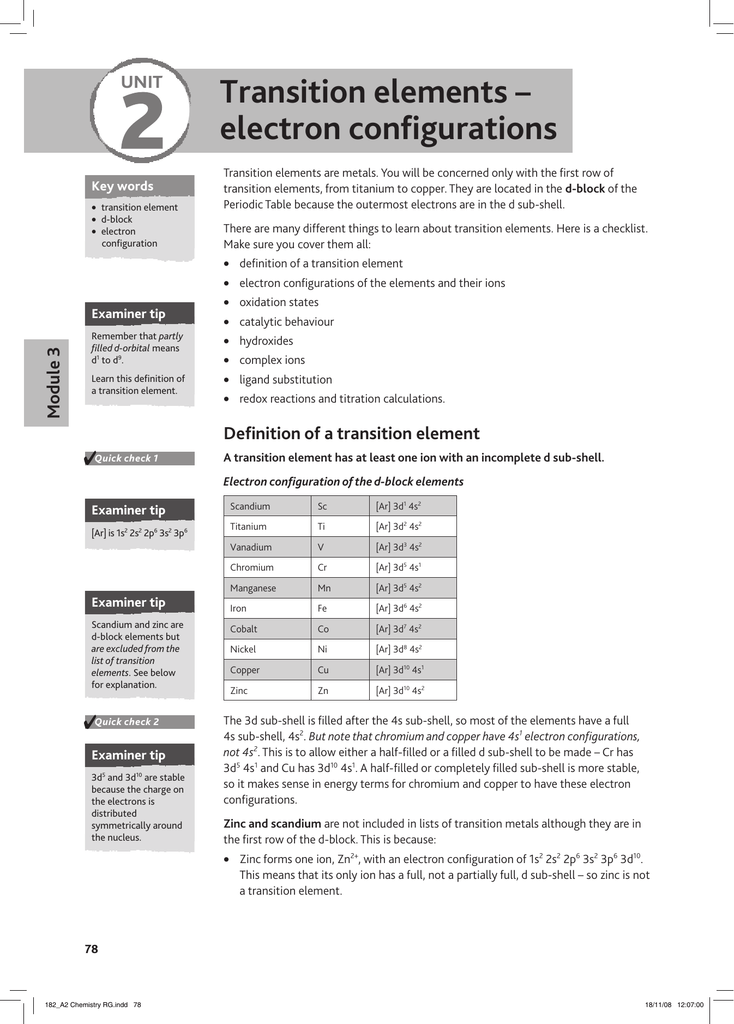
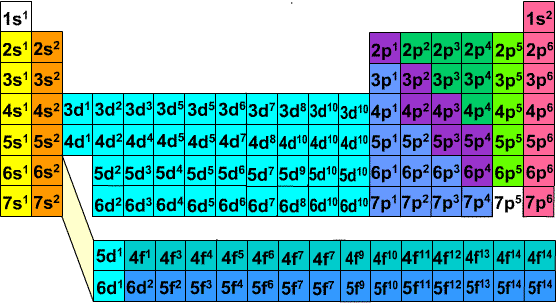
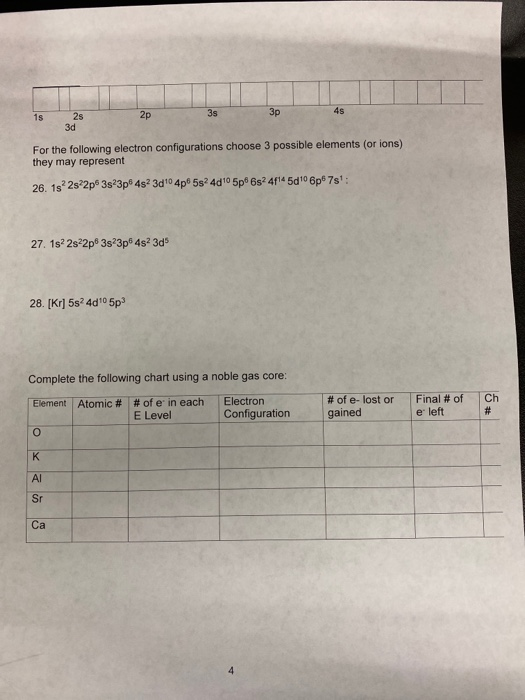
Electronic Configuration Of Ions Of 3d Elements
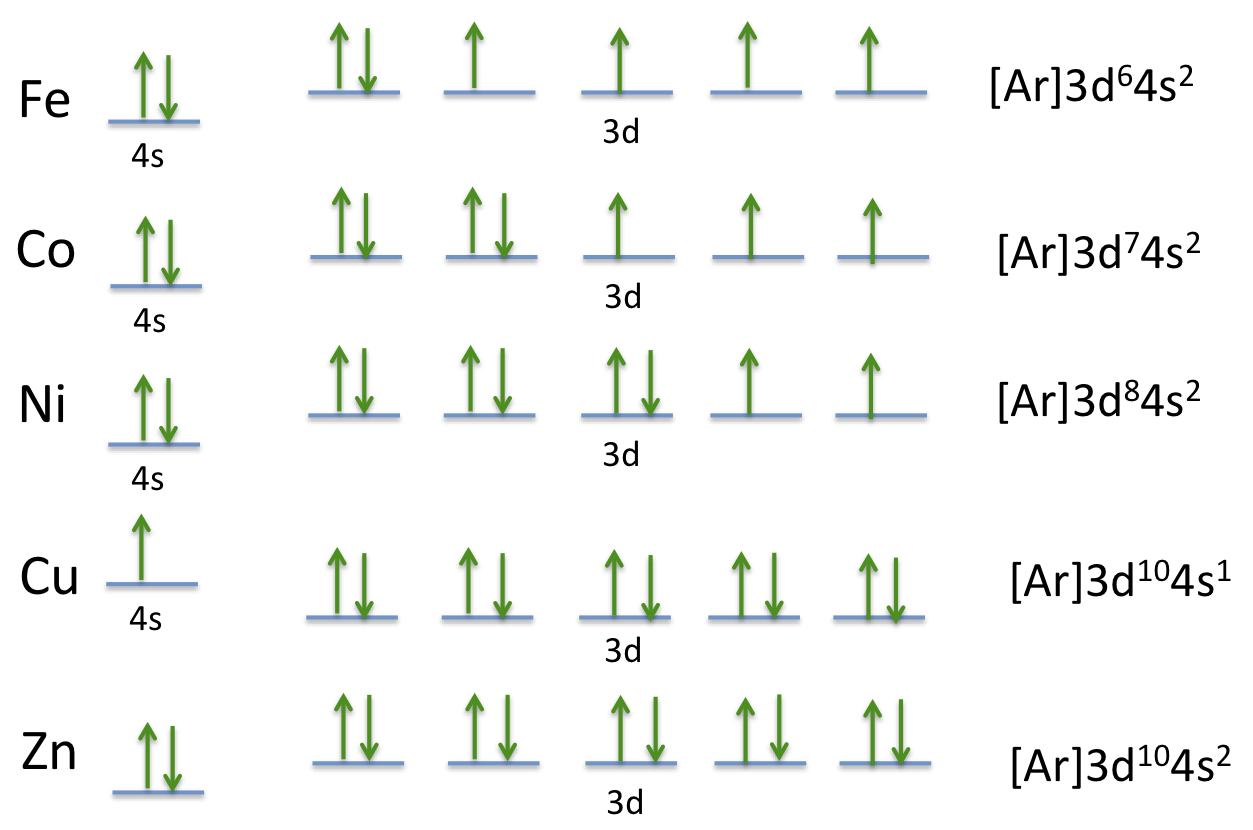
Each additional electron you add usually goes into a 3d orbital.

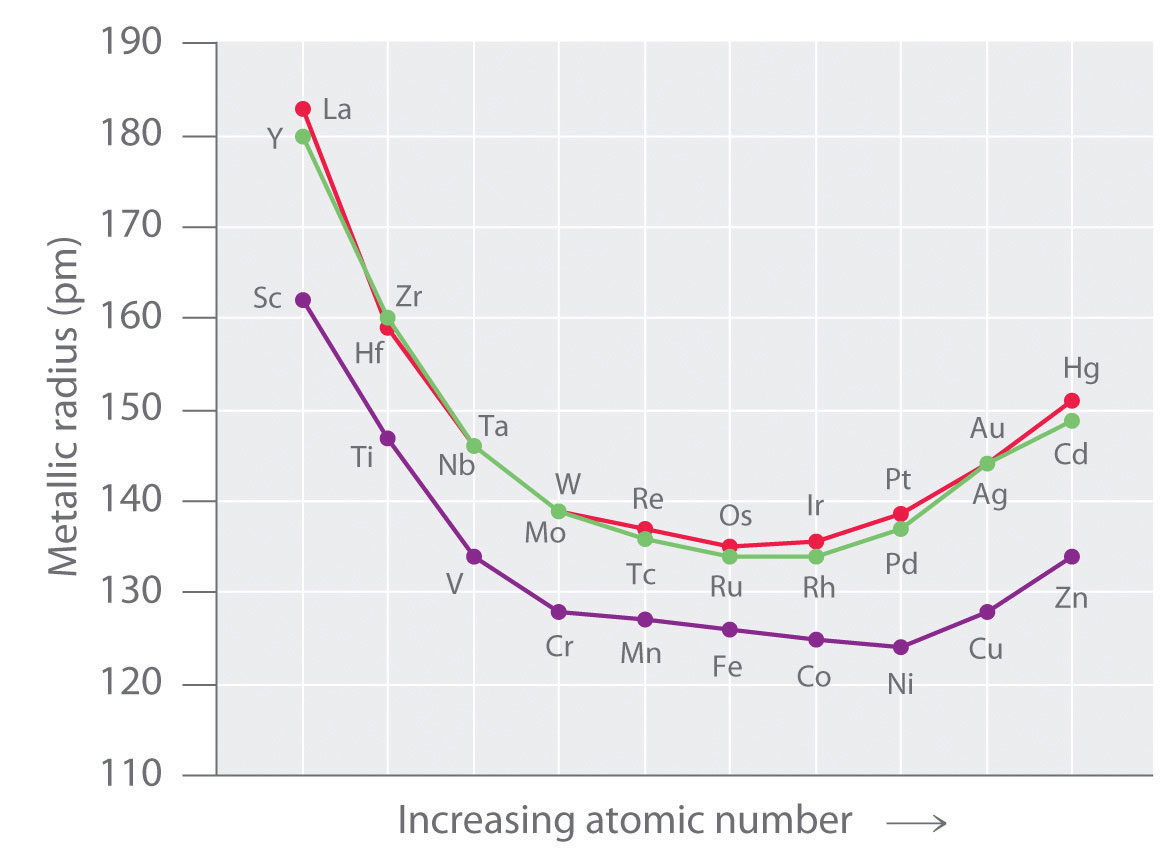
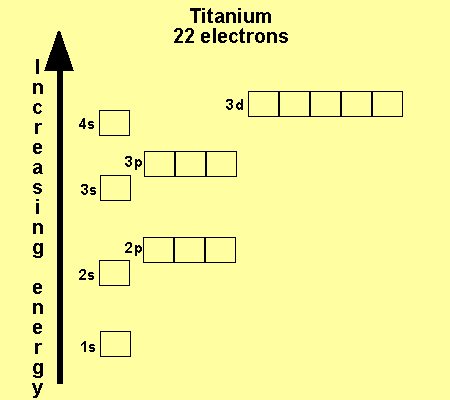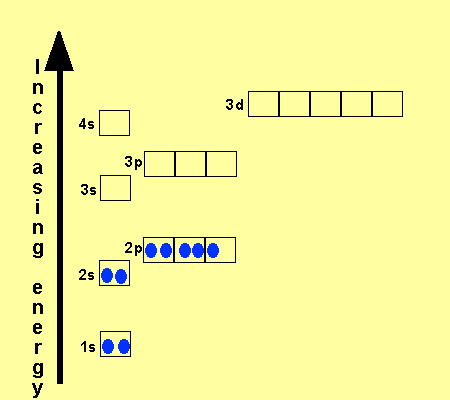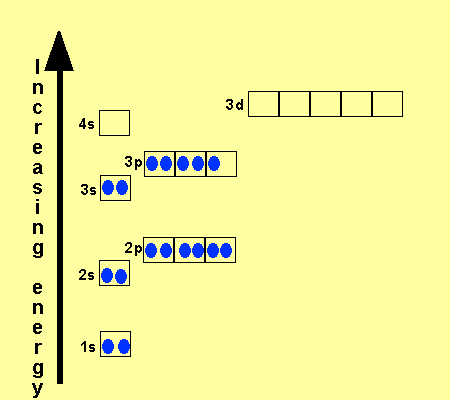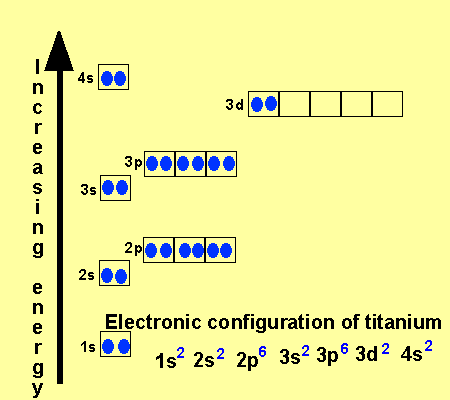
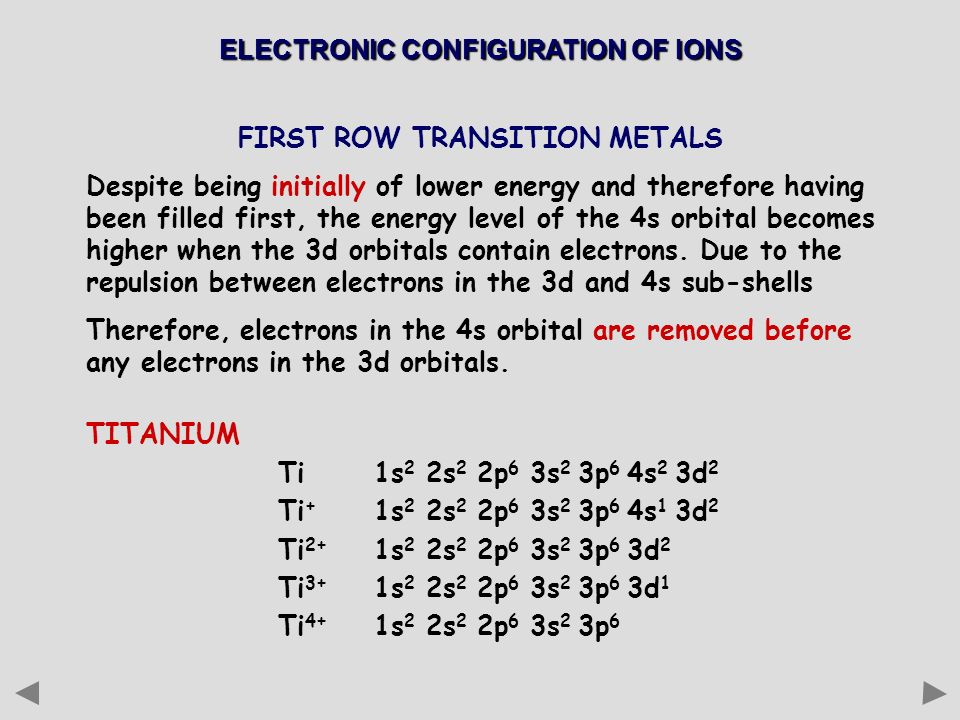
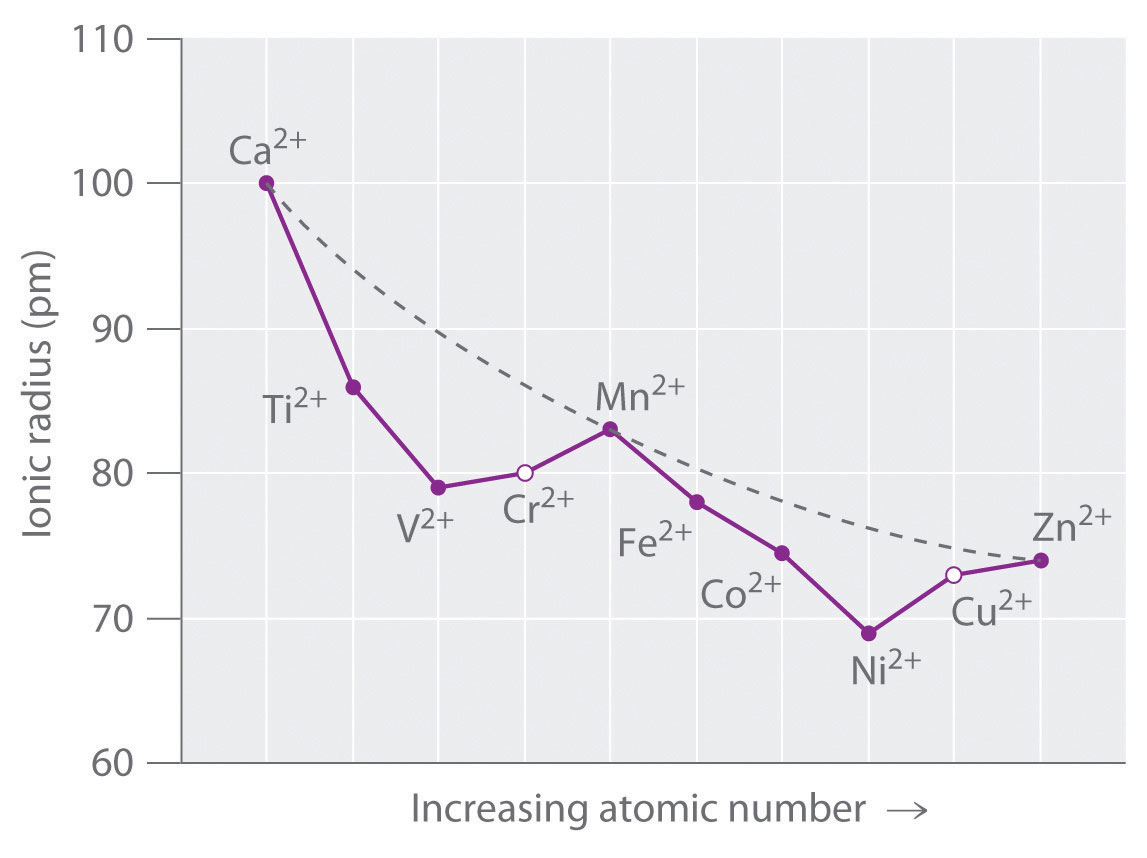
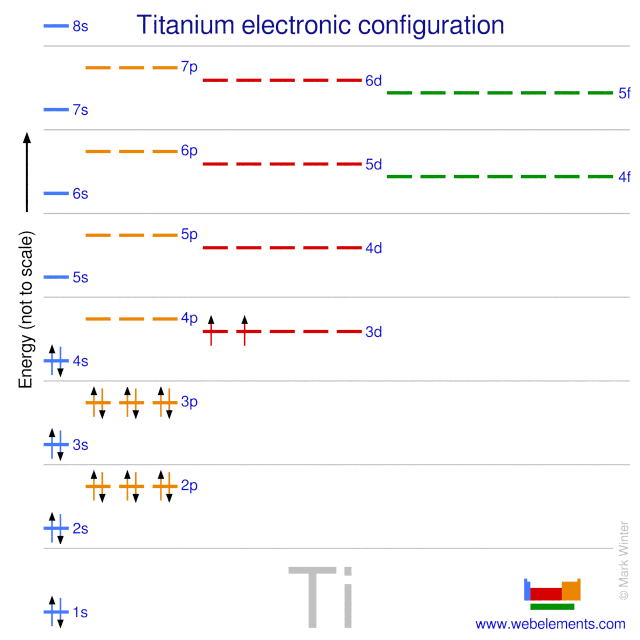
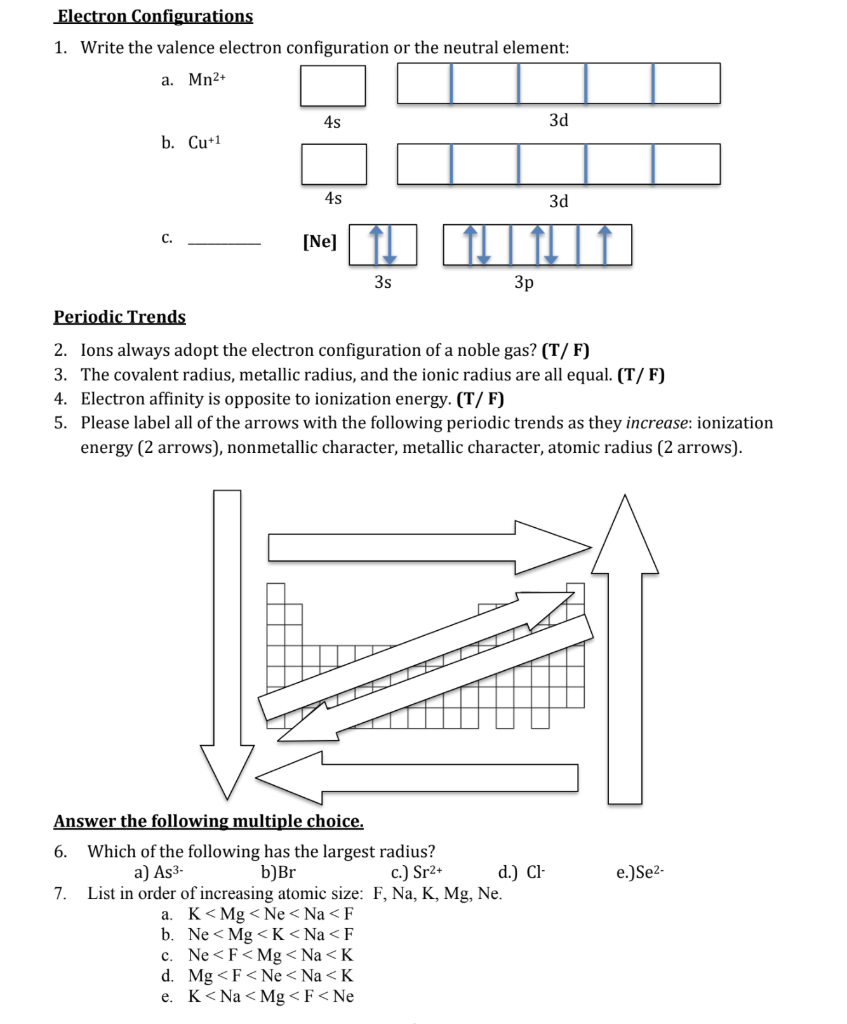
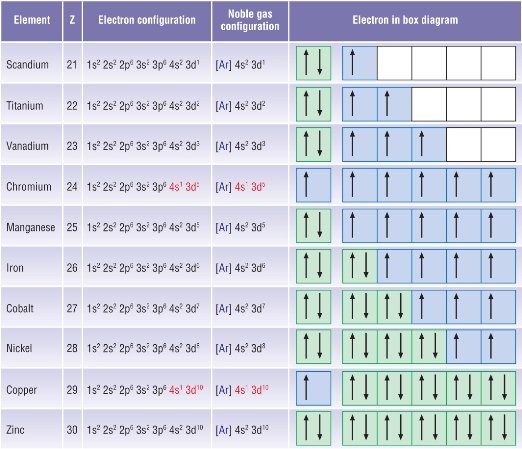
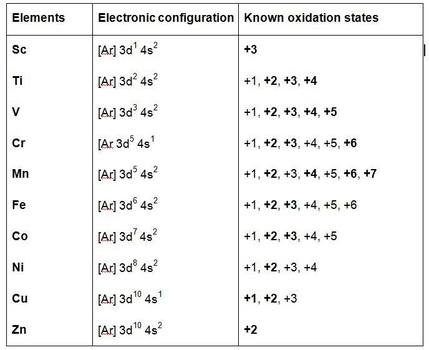
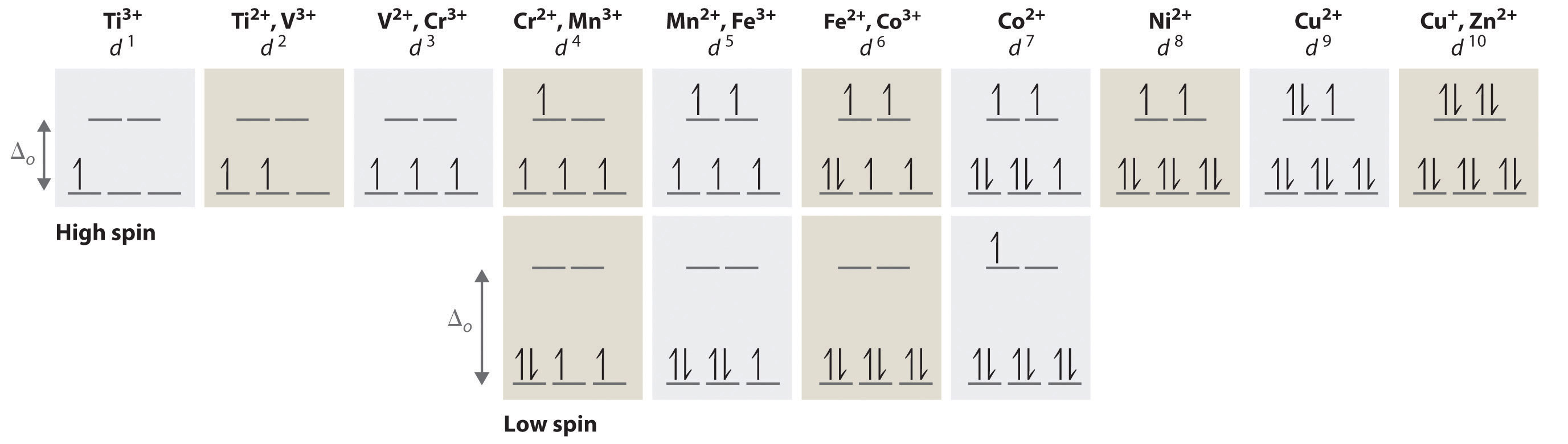
Electronic configuration of ions of 3d elements. Such an arrangement helps explain the periodicity and periodic trends observed across the elements of the periodic table. The electrons equal to the charge on the iob are removed from the neutral atom in case of positively charged ions. In our example this is the electron configuration of erbium. The electronic structures of the d block elements are shown in the table below.
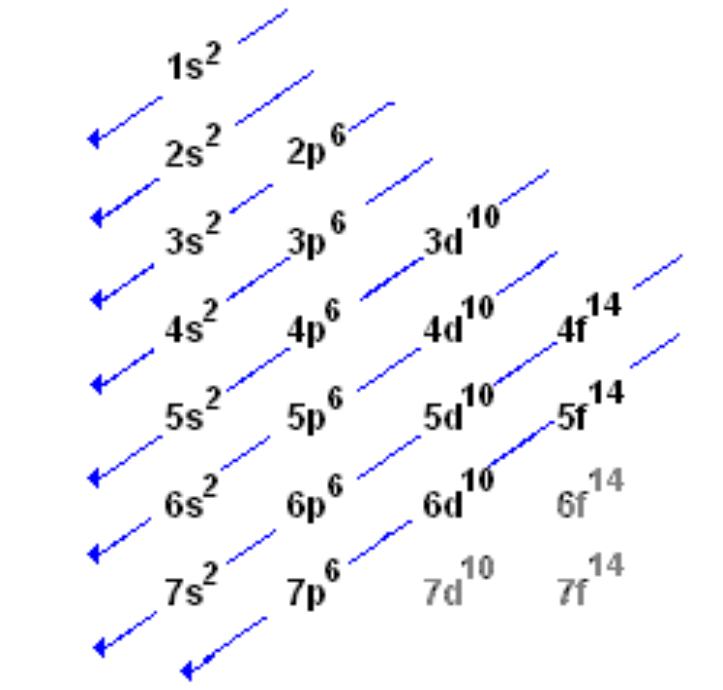
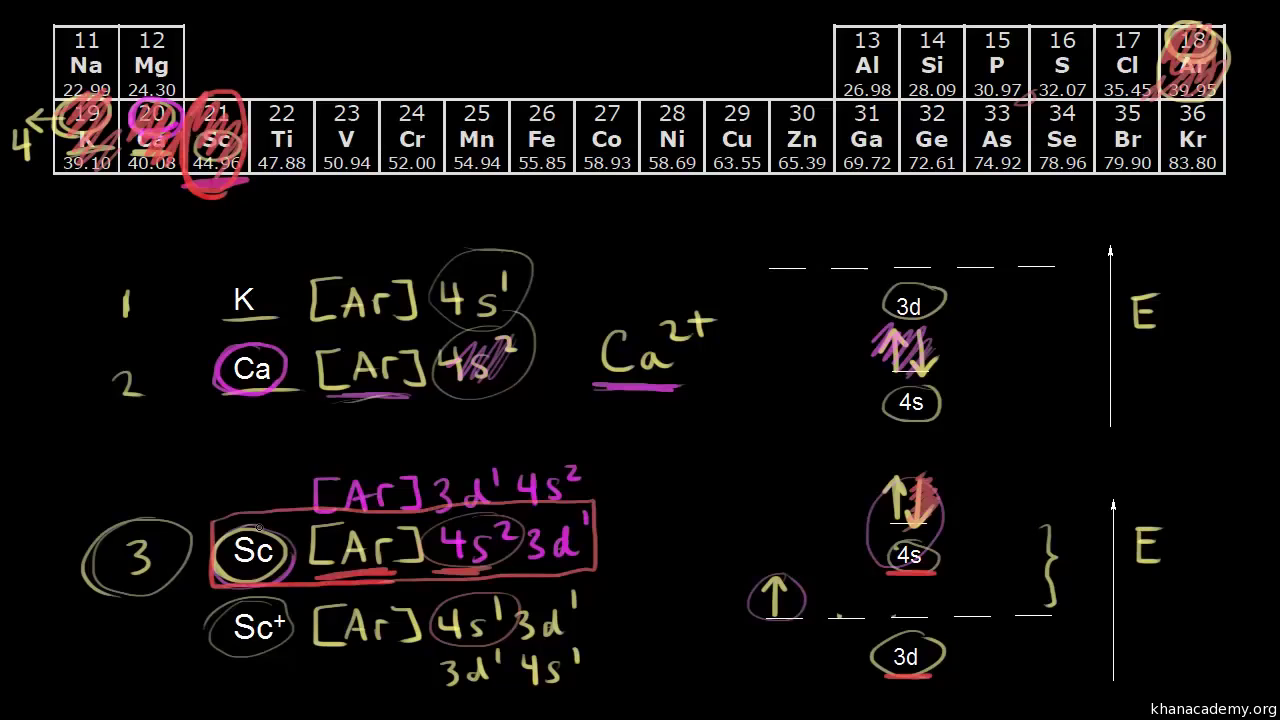
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 12 5s 2 5p 6 6s 2. From sc on the 3d orbitals are actually lower in energy than the 4s orbital which means that electrons enter the 3d orbitals first. The electronic configuration of the ions may be written almost similar to these of the atpms in case of negatively charged ionsthe extra electrons equal to the negative charge is added to the appropiate orbitals. Generally the electronic configuration of these elements is n 1 d 110 ns 12.
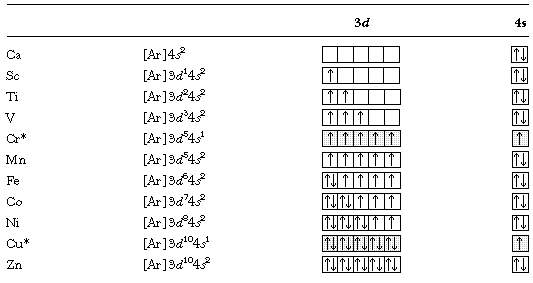
In the ground state the electron configuration of the transition metals follows the format ns 2 nd xas for the electron configuration for transition metals that are charged ie. When writing an electron configuration first write the energy level the period then the subshell to be filled and the superscript which is the number of electrons in that subshell. The n1 remains for the inward d orbitals which may have one to ten electrons and the peripheral ns orbital may have one or two electrons. Count elements that were not crossed out in each block column assigning 1 electron per element and write down their quantity next to the block symbols for each block column like this.
In this video well discuss this in more depth and walk through all of the electron configurations for the 3d. The n 1 stands for inner shell and the d orbitals may have one to ten electrons and the s orbital of the outermost shell n may have one or two electrons. I will come back to that later as well. It is helpful to first write down the electron configuration.
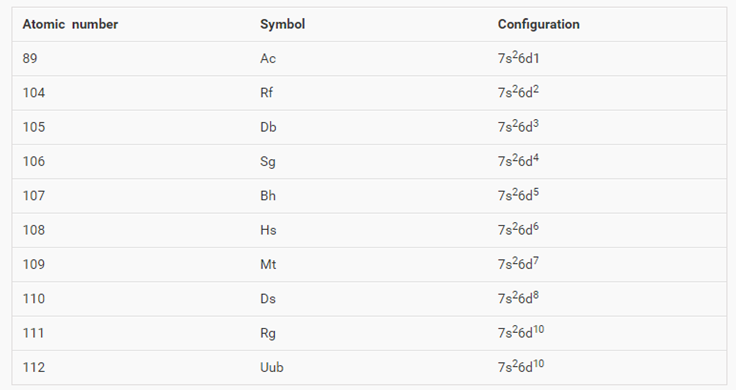
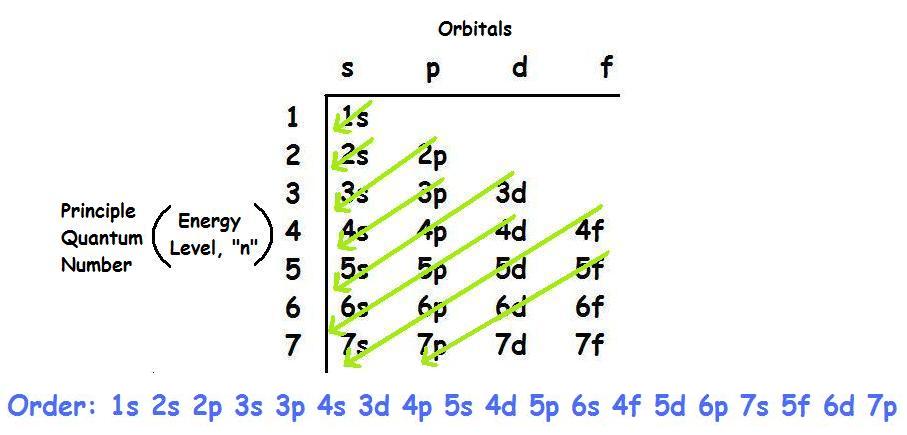
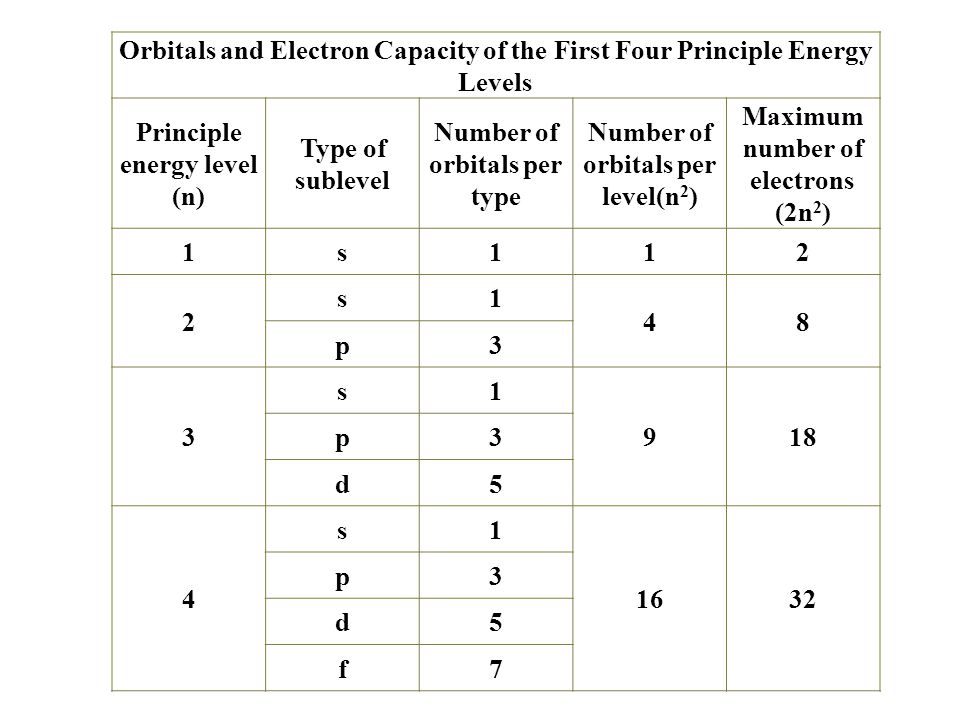
The aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals but this is actually not true for most elements. The m shell contains 3s 3p and 3d and can carry 18 electrons. The n shell containing 4s 4d 4p and 4f can carry 32 electrons. The rules above allow one to write the electron configurations for all the elements in the periodic table.
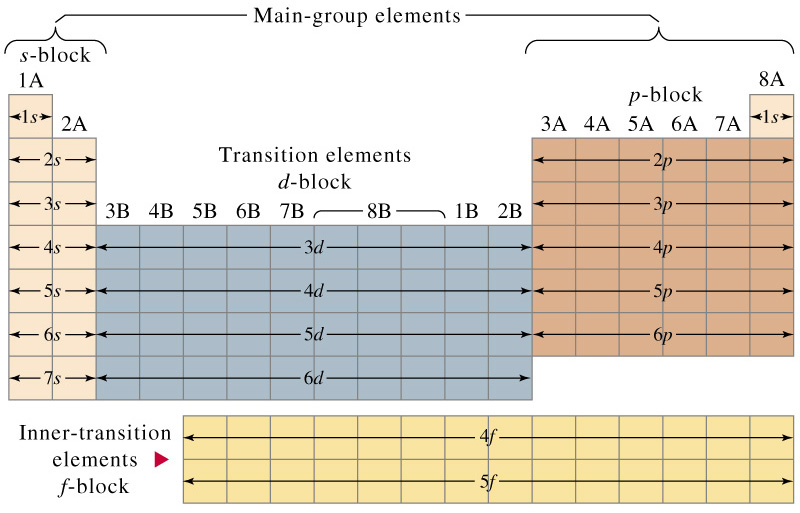
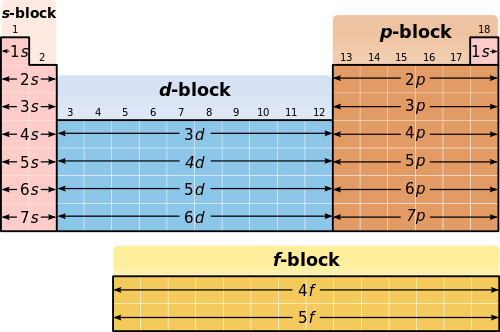
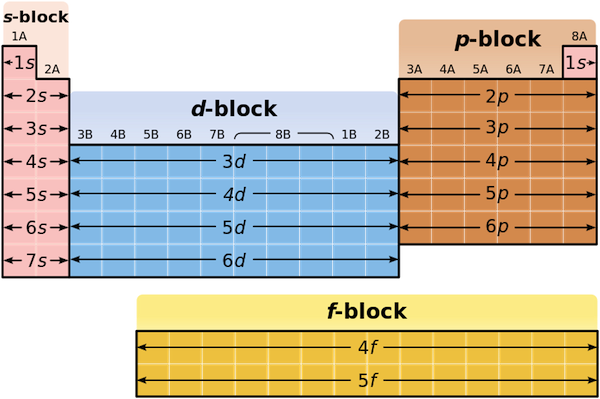
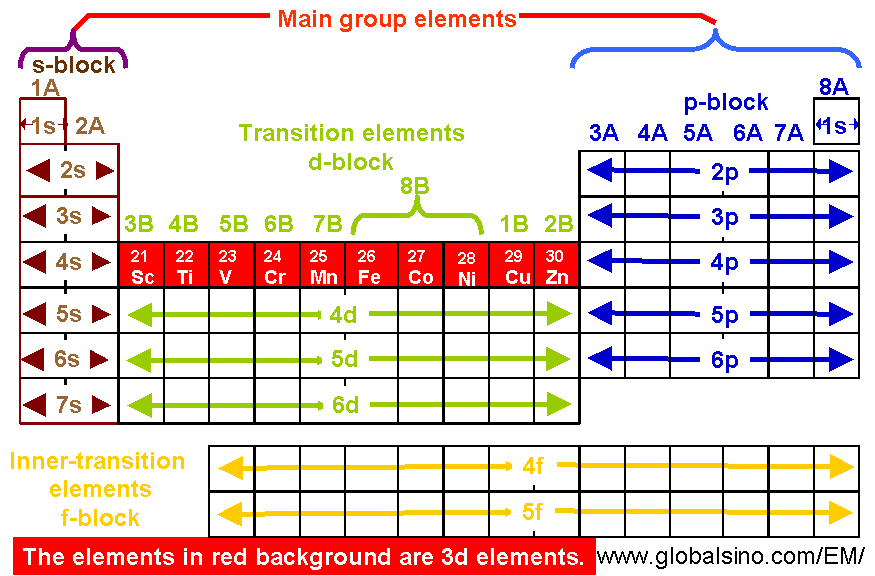
The d block includes the middle area marked by s and p blocks in the periodic table. Most chemistry books and chemistry teachers try to explain the breaks in the pattern at chromium and copper but not very convincingly. The general electronic configuration of transition elements is n 1d1 10ns1 2. This list of electron configurations of elements contains all the elements in increasing order of atomic number.
To save room the configurations are in noble gas shorthand. This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol.
_Oxidation_States_for_First_Row_Transition_Metals.jpg?revision=1)



















.jpg?revision=1&size=bestfit&width=656&height=425)